In recirculating systems, the pH value can fluctuate more than in run-to-waste systems. The same goes for the EC, ppm value. So it's wise to check the EC, ppm and pH levels regularly. But... AQUA nutrients are balanced in such a way that you don't have to adjust the pH.

An EC / ppm-meter can measure the concentration of dissolved salts and can also measure the total volume of nutrient elements that are dissolved. In recirculating systems it is certainly not to be trusted 100%. This is because certain nutritional elements have built up in the nutrient solution, while at the same time others have become diluted.
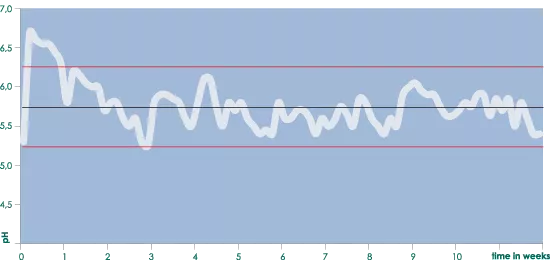
It is advisable to start with an EC that is between 0.8 and 1.0 higher than the EC (ppm values) from the water supply and to gradually raise this as necessary to a maximum of 1.3-1.7 above the water supply’s EC (ppm values). Keeping a regular check on the nutrient solution’s pH, EC and ppm levels and observing the plants is necessary to be able to take the correct action at the correct time. (If necessary), pH fluctuations between 6.2 and 5.2 are perfect. (see the graph ‘pH development using Aqua nutrient’).
Do not act too hastily!
Acidity (pH)
Stable pH values
Stable pH values are important for optimizing the availability of nutrients for the plants. If we compare run to waste systems, with recirculating growing systems then we will see that the pH value in the latter fluctuates more and should therefore be supervised more carefully. This fluctuation occurs because waste products from the roots directly affect the nutrient solution’s pH value. This influence is, among other things, dependent on the plants’ stage of development, their condition, the nutrient solution’s composition and the water supply.
During the growing phase, plants tend to cause the nutrient solution’s pH to rise. This happens because at this stage the roots can excrete relative large quantities of elements that increase the pH. During thefloweringphase,thereversehappens: the roots now produce acidic secretions causing the nutrient solution’s pH to fall.
To a large extent, the nutrient solution’s composition determines whether or not the roots excrete predominantly alkaline or acidic secretions. By using different nutrient solutions that are customized to the different phases of the crop (vegetative and generative), you ensure that the pH remains as stable as possible.
Trace elements
Trace elements present in the water also have an effect on the pH during cultivation. In hard water areas (high bicarbonate content) the nutrient solution’s pH shows a tendency to rise after the solution has been prepared and the pH balanced. By balancing the nutrient solution with a lower pH value (5.2-5.3), more bicarbonate is neutralized and the pH shows less tendency towards rising. In soft water areas with low bicarbonate content (osmotic water) drops in the pH value are more likely to occur. This is because soft water has less pH buffering capacity than hard water. This is also the reason why, in soft and osmotic water regions, nutrient solutions must be prepared with a higher pH (5.8-6.2).
If the pH is too low, certain nutritional elements such as iron and manganese, as well as the toxic aluminum, are dissolved more easily, which can cause damage as a result of over nutrient availability. If the pH drops too low it is sensible to raise it by using a caustic product containing bicarbonate. In doing this, you not only increase the pH, but also the nutrient solution’s pH buffer.
pH fluctuation with AQUA
Influencing pH
Plants are capable of actively influencing the nutrient solution’s pH. If the intake of food is disturbed when the plants suffer a pathogenic attack, for example mould, it can cause the nutrient solution’s pH to drop below 3. Another symptom can be noticed with iron deficiencies when the pH rises., In this case, the pH is actively lowered to make iron more available to the plants. For this and other reasons, it is not advisable to have the same value for the pH continuously. solution and a pH between 5.2 and 6.2 there should be no nutrient problems. If the pH should be lower than 5.0 or higher than 6.4 for a few days, it is advisable to carry out manual adjustments or to change the composition of the nutrient.
If CANNA Vega is being used and the pH drops too low during the short day cycle, changing to CANNA Flores nutrient is recommended (CANNA Flores is less acid; it is important to note that the plant now receives nutrient that is the best possible for its bloom).
pH vs Nutrient element availability

pH stable
With CANNA AQUA, every effort is made to ensure that the pH in the nutrient solution remains as stable as possible without affecting the quality of the nutrient.
In tests that where carried out in which the daily pH and EC were measured and complete weekly nutrient analyses were made, it was shown that the pH fluctuated between 5.2 and 6.2 throughout the complete cultivation cycle, (with the exception of the first few days). In between, it wasn’t necessary to correct the pH.









