In this article we will focus on how the plant takes up different nutrients and how deficiency symptoms (caused by substrate imbalance) can be recognized and solved.
Nutrient uptake and transport
Nutrients are usually taken up by the root system. This process involves the following steps. The first requirement is mobility of the nutrients through the soil or any substrate in the rhizosphere to the roots. Then the nutrients need to pass several ‘root barriers’; the cell wall, followed by the cell membrane. The crucial steps inside the plant are the migration of the nutrients to vascular tissue (called the xylem), followed by cell to cell transport.
The shifting of nutrients through the soil depends on the several soil characteristics; pH, structure, moisture content and microbial activity. Some microorganisms affect the rhizosphere (especially mycorrhizal fungi, which interact directly with plant roots), but most soil borne microorganisms do not or hardly affect the rhizosphere. Microorganisms can be beneficial (e.g. improving nutrient availability) or harmful (e.g. in competition for soil nutrients or causing root diseases). The dissolved nutrients are transported with the convective flow of water from the soil to the plant roots. This flow depends highly on the water consumption of the plant and the average nutrient concentration in the water. As we will see later in this article, water uptake by the plant and nutrient content in the substrate can be controlled easily by the grower.
A small percentage (less than 1%) of the nutrients is taken up by interception through the root tip. This interception is based on direct ion exchange, where positively or negatively charged elements are swapped (e.g. a proton (H+) from the root for a potassium ion (K+) from the substrate or nutrient solution).
Now how are the nutrients ‘in the flow’ taken up by a plant (Figure 1)? The biggest barrier is usually the cell membrane which is highly selective. The basic structure of a cell membrane is the phospholipid bilayer, which has very low permeability for most nutrients. Carbon dioxide, oxygen, water and some neutral molecules like urea are the only products that can easily pass the membrane directly through the lipid layer by diffusion.
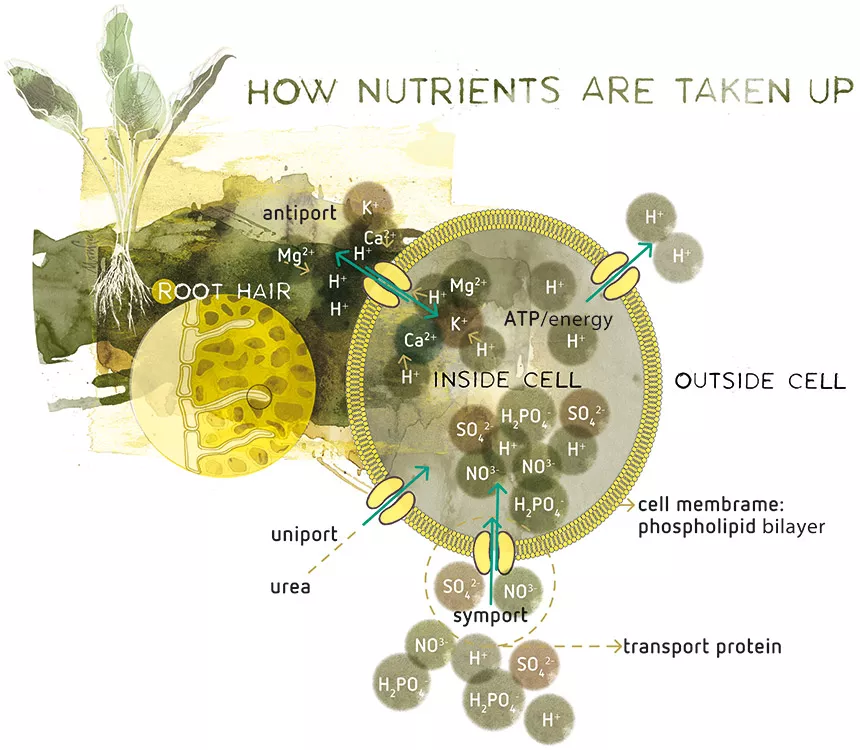
All other essential mineral nutrients are absorbed as ions (except boron). Therefore, all nutrients (except boron) need membrane transporters. To facilitate the uptake of these nutrients, the cell membrane contains so-called transport proteins embedded in the cell membrane, controlling the intracellular environment (=the space in the plant cell). Two main mechanisms of cross-membrane movement can be identified: passive and active. Passive movement is the easy way, via carrier proteins and transport through ion channels. The active method is by ATP-ases or cotransport (Knox, Ladiges & Evans). ATP-ases are proteins that carry the ‘energy molecule’ ATP. The energy that is released by breaking down ATP is used to open or close specific transportation ports.
The driving force for passive movement is diffusion. It is selective for single nutrient molecules, requires no input of energy and has a non-linear dependence on concentration. Molecules will diffuse until the concentration is the same everywhere.
Ion channel transport can be controlled by voltage or ion concentration, even by light or hormones. Active transport requires input of energy, but nutrients can be transported against their concentration gradient.
Long distance transport
Several pathways of nutrient transport have been described in the plant. The most common one is the nutrient uptake by the root, followed by ‘long distance transport’ via the xylem vessels to the leaves and flowers (or any other plant organ). For the long distance transport two driving forces play a key role; the water potential gradient and the root pressure. Root pressure arises when osmosis drives water from the soil into the roots. This is effectively because plants accumulate the nutrients taken up in the xylem tissue.
Factors that influence nutrient uptake
There are intrinsic and environmental factors which affect the nutrient uptake. Charge and ion diameter are intrinsic factors. Environmental factors are light, temperature, water, O2, pH, concentration and interaction between nutrients. The uptake often increases in the following order; uncharged molecules are taken up better than monovalent cations (ions with a +1 charge) and anions (ions with a -1 charge), followed by bivalent cations (ions with a +2 charge) and anions (ions with a -2 charge) (Marschner, 2011).
The nutrient uptake is pH dependent, but not every nutrient is equally affected. In most cases there is an optimum and too high and too low pH levels decrease the nutrient uptake. The root zone pH influences the charge of the root surface which is slightly negative. Most nutrients are plant available in the pH range 5.5-6.5. Light is a source of driving forces, therefore, there are diurnal fluctuations in nutrient uptake. Like pH also temperature has an optimum level, too high and too low temperatures decrease nutrient uptake.
Temperature is a driving force for evaporation of plants and in turn opening stomata and the root zone temperature affect nutrient uptake. Water is important, because, except for interception, nutrients are transferring through mass flow and diffusion, all of which are dissolved in the soil solution. It is important to maintain a suitable soil water content (60-80% field water holding capacity) for optimal growth of plants. Oxygen will limit plant growth in poorly aerated substrates (Hopkins, 1950). The higher the nutrient concentration the higher the uptake rate, the increase slows down at very high concentrations. Interaction between ions can be antagonism (competition) or synergism. In the first case one ion inhibits the absorption of another ion. And in the second case one ion enhances the absorption of another ion.
Competition can be between cation versus cation, anion versus anion, the same charge and different charge. A single salt resulting in plant poisoning is called toxicity of a single salt (KCl, CaCl). Ion antagonism is referred as the interaction among ions and can limit the toxicity of a single salt (NaCl+KCl+CaCl2 or NaCl+CaCl2). Synergism is that anions promote cation uptake and divalent cations promote mono-charged cations (Ca2+ promotes the uptake of K- and Cl-).
Fertilization of crops
The uptake of nutrients is facilitated by cell membrane bound protein transporters membrane transporters. Synthesis of transporters responds to nutrient deficiency and toxicity. Nutrient transporters behave like enzymes. Transport can be driven by concentration and electrical gradient (passive transport) and metabolic energy (active transport).
The quality of the crop, or the fertilization success of crops depend on a phenomenon, which can be shortly explained by the following theory. The barrel theory (or the law of minimum) is about the crop yield which is limited by the most deficient nutrient, and the limited nutrient is changeable. This concept was originally applied to plant or crop growth, where it was found that increasing the amount of plentiful nutrients did not increase plant growth. Only by increasing the amount of the limiting nutrient (the one scarcest in relation to ‘need’) was the growth of a plant or crop improved (see figure 2).

There is also absorption of mineral nutrients by the leaves. Foliar nutrition is a method in which the fertilizer is applied to plant shoot, usually to leaves. The advantage of foliar nutrition is that the supply and uptake of nutrients is effective and fast, because the usual lag period between root uptake and vessel transportation towards the plant organs is cut out. Furthermore, the nutrients applied have a higher utilization rate. Leaf fertilizers are usually a supplement way to compensate macronutrient deficiency such as nitrogen and magnesium, but also effective against micronutrient deficiencies such as iron.
The concentration which can be applied is not too high. The nutrients need to remain on the leaf surface, preferably in a thin film. Therefore, the mixture of nutrients should be applied together with a surfactant. Spraying is recommended only in the evening or on a cloudy day to prevent burn marks.
Deficiency symptoms caused by substrate imbalance
We have seen the driving forces behind the nutrient uptake. Plants can be very selective, but they can never run away in search of food. Despite the tricks that plants developed during evolution, they surely need the growers’ help. For a grower it may sound very easy to identify individual deficiency symptoms. We observe our plants and try to answer as many of the following questions as possible. Do we see necrotic spots? Do we see yellowing of the leaves? Where do these symptoms occur? In the older plant parts, or just in the younger? Important questions to exclude elements and shorten the list of suspects.

Unfortunately, deficiency symptoms are often caused by external factors, not the amount of fertilizer we supply to our plants. A very common issue here may be over watering (Figure 3). The substrate is too wet, leaving less room for air. New plant roots definitely need oxygen which they take up from these air spaces. In case of oxygen stress, roots will die – a process that occurs faster than you may think. Root tips die off and nitrogen and potassium are amongst the first nutrients whose uptake are reduced. If the substrate remains too wet, the plant growth is lagged and symptoms much like nitrogen deficiency arise.
To prevent over watering, check your substrate regularly. In case of a wet substrate, check drainage and reduce the amount of water given per turn. In case of over watering, skip one or a few turns. Allow the substrate to dry a little. This will stimulate the plant to form new roots to replace the ones that died before.
As we have seen in the previous paragraphs, the plant itself influences the soil pH. Aiming for a neutral ion charge inside and outside the plant, nutrients like ammonium (NH4+) are taken up in exchange of a proton (H+). As H+ builds in the soil, this may lead to acidification of the substrate. Nutrients like phosphorus, potassium, sulfur, calcium, magnesium and molybdenum (although abundant in the rhizosphere) will become less available for the plant.
The risk of acidification of your substrate is reduced considerably if you choose the right substrate. Do not use improper peat mixes, check the soil pH before use; at pH values lower than 5.5 the risk of acidification is increased considerably. Also, keep in mind that peat based substrates that you want to re-use may have used up the limestone buffer during a previous cultivation cycle. A limestone buffer can be ‘repaired’ by mixing some additional limestone prior to planting and will reduce the chance of deficiency symptoms caused by low pH values.
On poorly buffered substrates or inert substrates like rock wool, we always advise to set the pH value of your nutrient solution to values ranging from 5.2-6.2 on rock wool to 5.8- 6.2 on peat mix. Rock wool, or any other inert substrate does not have a nutrient or pH buffer. If the nutrient solution is not applied at the right pH range, nutrient deficiencies caused by a high pH may occur. Deficiency symptoms that can be seen include deficiencies of trace elements like iron, copper, zinc and manganese.
In case of high soil pH values, one could choose to decrease the pH value of the nutrient solution. During crop cultivation, the addition of some extra ammonium may help. This nitrogen source helps to lower the pH around the roots, which increases the plant availability of most trace elements.