It’s a well-known fact that plants need sufficient nutrients to grow and that these nutrients need to be applied in the right proportions. Too much or not enough of one or several nutrients may interfere with the plant’s development.
Sometimes reduced growth is not caused by a shortage of the element in question, but by environmental factors that may play an even more important role. In this article, we are going to look specifically at the effect of the ammonium/nitrate ratio and its effect on the growth and development of the final crop and environmental factors like temperature, root zone pH and soil bacteria that may alter ammonium and nitrate availability.
Nitrogen is the building block of amino acids, proteins, enzymes and chlorophyll. Plants can absorb nitrogen either as nitrate (NO3-) or ammonium (NH4+), and so the total uptake of nitrogen usually consists of a combination of these two forms. It’s no surprise, then, that the ratio between these two forms of nitrogen is highly significance, and affects both the plants and the medium.
For optimal uptake and growth, each plant species may require a different ratio of ammonium to nitrate. As we will see, the correct ratio also varies with temperature, growth stage, pH in the root zone and soil properties.
Nitrogen metabolism in plants
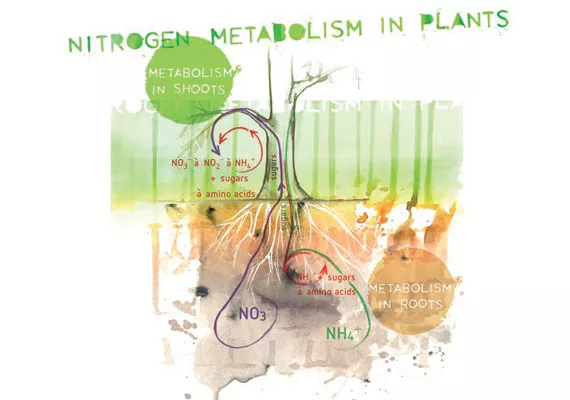
To better understand the effect of nitrate and ammonium uptake by the plant, we need to understand the different ways that these two forms of nitrogen are metabolized.
In most plant species, both the roots and shoots can convert the nitrates taken up by the plant; first into nitrite and then into ammonium. These processes are controlled using enzymes. Whether the nitrate is metabolized in the roots or shoots depends on several factors, including the amount of nitrate supplied to the roots and plant species. When levels of nitrate are limited, it is quickly metabolized in the roots. When there are greater proportions, the nitrate is transported to the shoot and metabolized there.
The intermediate product nitrite is highly reactive and potentially toxic to the plant. It is therefore quickly transported to specific parts in plant cells to separate the nitrite from other vital processes in the cells. These parts are plant cell organelles called plastids. They can be found in almost every cell in the plant, from the roots to the top leaves. In the roots, plastids are often used for sugar storage. In leaves, the most common plastids are the chloroplasts where the process of photosynthesis takes place. Nitrite is converted to ammonium in the plastids.
The conversion of nitrates to ammonium that occurs in the leaf is a process fueled by solar energy, which makes it an energy-efficient process. However, ammonium in the roots has to be converted into organic N-compounds first. This process is fueled by carbohydrates and thus occurs at the expense of other plant life processes, such as plant growth and fruit production. These sugars have to be delivered from their production site in the leaves, down to the roots first.
The final step in nitrogen metabolism is the relatively rapid conversion of ammonium into glutamate, a major amino acid which can be used as a source for other amino acids and as a building block for proteins and enzymes.
The effect of temperature on the absorption of nitrogen
Higher temperatures usually increase the metabolism of plants and hence its energy use. This process is also known as respiration. Sugars are consumed faster, making them less available for metabolizing ammonium in the roots. At the same time, at high temperatures, the solubility of oxygen in water decreases, making it less available as well. Thus at higher temperatures, a lower ammonium/nitrate ratio seems an obvious choice.
At lower temperatures, ammonium nutrition may be a more appropriate choice, because oxygen and sugars are more available at root level. Additionally, since the transport of nitrate to the leaves is restricted at low temperatures, fertilization based on nitrates will only delay the growth of the plant. The effect of the substrate temperature also depends on plant species.
Plant species specific nitrogen uptake
When ammonium levels are higher, sugars need to be transported down from the leaves to the roots to metabolize the ammonium. In flowering and fruiting plants, such as tomato and cucumber, and plants where the majority of the growth is in the leaves (e.g. cabbage, lettuce, spinach), sugars are consumed quickly near their production site and are much less available for transport to the roots.
In this case, ammonium will not be efficiently metabolized and the use of a lower ammonium/nitrate ratio is preferable.
The effect of the ammonium/nitrate ratio on pH in the root zone
The electrical balance in the root cells must be maintained, so for each positively charged ion that is taken up, a positively charged ion is released into the soil and the same is true for negatively charged ions. This means that when the plant takes up ammonium (NH4+), it releases a proton (H+) to the soil solution. An increase of the proton concentration around the roots decreases the pH around the roots (more acidic).
Similarly, when the plant takes up Nitrate (NO3-) it releases bicarbonate (HCO3-), which increases the pH around the roots (more alkaline).
The effect of ammonium and nitrate uptake is especially important in soil-less media, where the roots can affect the pH of the medium more quickly because their volume is relatively large compared with the volume of the medium. To prevent the pH of the medium from changing too rapidly, an appropriate ammonium/nitrate ratio and substrate temperature are essential, according to the plants growth stage.
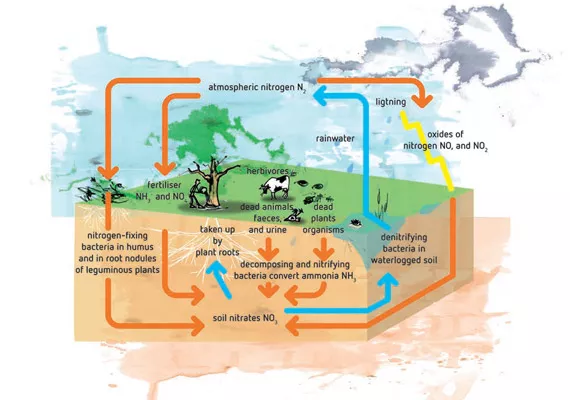
Nitrogen conversion processes in the soil

As explained earlier, ammonium uptake usually makes the soil pH in the root zone fall, while nitrate uptake raises the soil pH. Under certain conditions, however, the pH may not respond as expected due to microbial activity around the roots. Most of the processes that involve ammonium and nitrate are part of the nitrogen cycle (figure 2).
The most important step is the biological oxidation of ammonium to nitrate, known as nitrification. This process consists of various steps and is mediated by autotrophic, obligate aerobic bacteria, meaning that oxygen is required. The plants take up their nitrogen source as nitrate rather than ammonium, effectively increasing the pH in the rooting zone.
The nitrification process can easily be disturbed, and such disturbances usually result in ammonium accumulation in the soil. One of the causes is a low soil pH, which limits the nitrogen conversion by depressing the microbial oxidation of ammonium.
Secondly, as mentioned earlier, converting ammonium to nitrate in the soil requires oxygen. In very wet soils, the air content drops which often means less oxygen available in the soil. In the absence of oxygen, microbial activity is usually low, meaning less ammonium is converted to nitrate and an accumulation of ammonium.
The soil micro-organisms need organic matter (dead plant material, humus) as a source of carbon. In poor soils with little organic matter like sandy soils, microbial growth and thus nitrification is limited. A low soil temperature can also depress nitrification, due to low soil micro-organism activity.
Achieving an optimum nitrate / ammonium ratio in hydroponics
In hydroponics, the standard quantities of NH4+ added to nutrient solutions for soilless culture make up between 5 and 10% of the total N supply, they will seldom exceed 15%. For roses this tends to be around 25% during the vegetative stage, whilst for melons it tends to be 0% during fruit development, for example. The fine-tuning of the levels of NH4+ supplied during crop growth simply occurs in relation to the pH levels in the root environment. The addition of NH4+ lowers the pH in the root environment, because of the boost to the cation (NH4+) uptake and a reduction in the anion (NO3-) uptake. When NH4+ is taken up, the plant releases H+ in order to maintain the plant’s electrical neutrality, which causes a lower pH in the root environment. Optimum pH levels in the substrate solutions range from 5 to 6 for almost all crops.
As explained earlier, the addition of NH4+ as a replacement for NO3- in substrate systems can reduce the uptake of other cations, like K+, Ca2+ and Mg2+, which can be explained by cation competition between NH4+ and these cations. The extent of this effect depends on various factors such as the crop, the growing conditions and the adjustments made in the ionic balance of the nutrients. Careful use of NH4+ is therefore recommended for crops which are sensitive to calcium deficiency. This is especially true when such crops are grown under climatic conditions that reduce the calcium transport to fruits. Good examples of this are the production of tomatoes and sweet peppers under dry and hot conditions. Both crops are sensitive to blossomend rot, caused by calcium deficiency in the fruit, which is stimulated by a hot and dry climate. Under such conditions every reduction in calcium uptake becomes dangerous and this includes the use of NH4+ too.