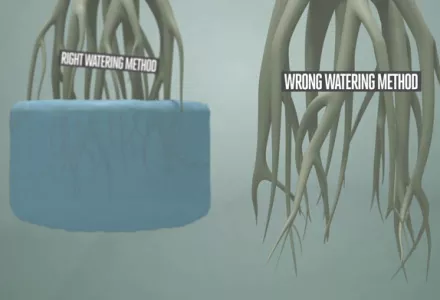
It is perhaps one of the most overlooked aspects of growing, but pH is very important (read: pH acidity: what it does to your plants) in hydroponics and soilless substrate horticulture. Soil has a good capacity for buffering pH. This means that even if the pH level of the water is not ideal, the make-up of the soil acts as a buffer and balances out the pH of the incoming water to a level that is generally acceptable. However, when you are growing without soil, in a hydroponic system for example, it is important to adjust the pH of the hydroponic nutrient solution because there is no soil to act as a buffer and correct the ph level if it becomes unbalanced.
pH is measured on a scale of 1-14, with 7 being neutral. Acids are lower than 7 and alkalis (bases) are above 7. There are plants, such as heather or rhododendron, that thrive at lower ph levels (pH 4.2 – 5.0), while other plants that prefer an alkaline growing environment with a much higher pH (>pH 7). In general, though, most plants prefer a slightly acidic growing environment. Also read part one: An introduction to pH
The plant can influence the soil life in its rhizosphere
For any plant’s roots to be able to absorb nutrients, these must be dissolved in a solution. If the pH is not at the right level, the plant will lose its ability to absorb some of the essential elements required for healthy growth. Most minerals and nutrients are more soluble – and thus more available to plants – in slightly acidic solutions than in neutral or slightly alkaline solutions. If the pH is too high or too low, nutrients become insoluble and precipitate out.
The process of precipitation (the reverse of dissolving) results in the formation of solids in the nutrient solution, and means that the nutrients are no longer available to the plants. Not all the precipitates sink and settle at the bottom of the feed tank. Some precipitates are suspended in the liquid and are so fine that they are invisible to the naked eye.
Once the nutrients have precipitated out of solution, your plants can no longer absorb them and will suffer from nutrient deficiency. Eventually they will die if this deficiency is not corrected. Apart from the problem of individual nutrient precipitation due to an incorrect pH, there is also the added problem of nutrient interaction, which can cause one or more nutrients in a fertilizer solution to become unavailable.

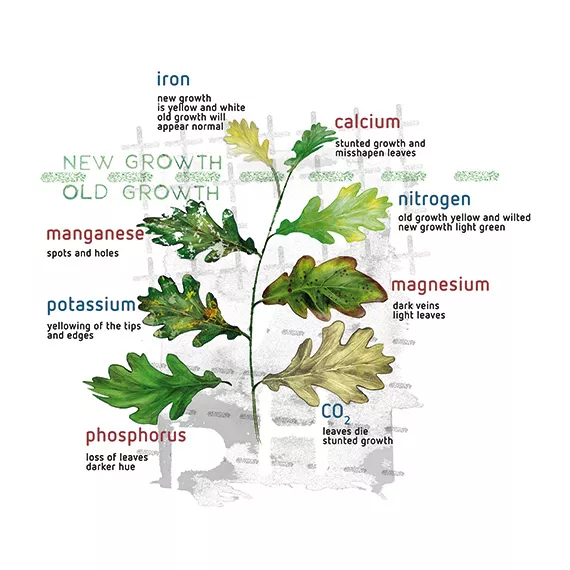
Plants let us know their problems (e.g. iron deficiency) through leaf symptoms, but by this time unfortunately it is too late. Although it is only required in very small amounts, iron is one essential plant nutrient whose solubility is greatly affected by pH, which is why it is added in a chelated form (or daily). Iron deficiency symptoms occur easily. At pH values of over 7, less than 50% of the element iron is available to plants. At pH 8.0, nothing is left in solution due to precipitation of the iron as iron hydroxide, Fe(OH)3 - which eventually converts to rust.
As long as the pH is kept below 6.5, over 90% of the iron is available to plants. By adding iron in chelated form, the sensitivity of the iron to pH levels is lessened, depending on the chelate form. For example, the chelator EDDHA keeps the iron available in a pH range of 4-9, while DTPA only works between pH 3.0 and 6.5.
Iron chelated with the more expensive EDDHA is often referred to as ‘red iron’, while iron chelated with the cheaper DTPA is called ‘yellow iron’. Every nutrient deficiency has its own way of manifesting itself in discoloration or malformation of the leaves. You can read more about nutrient deficiencies and macro- and micro-elements, in our Deficiency Guide.
How often do you need to check the pH of your solution?
The pH of the nutrient solution tends to rise over time, either due to nutrient uptake by the plant or, more importantly, through the diffusion of gases. As a result, pH needs to be checked periodically and adjusted if necessary. To start out, check the pH on a daily basis. Each system will change pH at a different rate and this will depend on a variety of factors. The type of growing medium used, the weather, the kind of plants and even the age of the plants all effect pH variations in both soil and fertilizer solutions (like BIOCANNA).
Once you have become familiar with the pH levels and the changes that occur in your system, you can start to do a less frequent pH check. Measure the pH of your tap water (this should be stable), the feeding-water and the soil. Note the results of these measurements and, if necessary, the adaptations you have made. In this way you can learn from your experiences and you will not have to measure so frequently in the future. Keep a close watch on the plants, too. If you see any abnormalities in the growth, the shape of the leaves or the colors of the leaves, check whether this might have been caused by a nutrient deficiency as a result of a pH that is too low or too high.
How can you check the pH and what are the advantages and disadvantages?
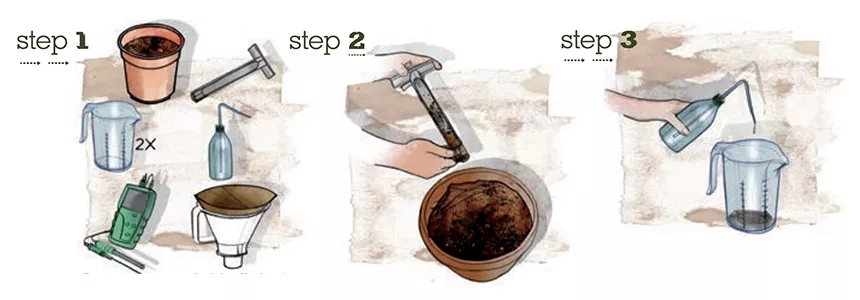
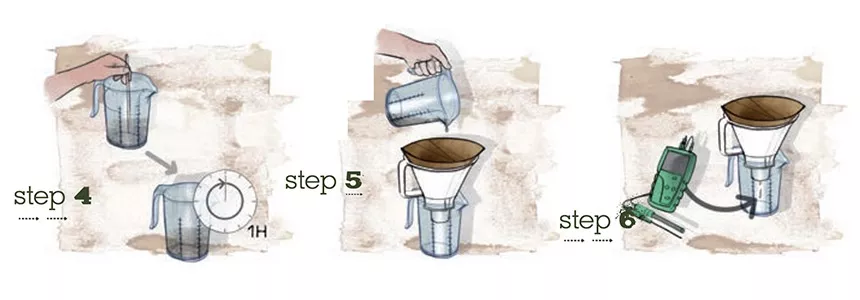
There are several ways to check the pH of the nutrient solution in a hydroponic system or in soil. The pH of soil can be checked with a pH-meter or with pH-indicators either directly in the soil or by testing a soil sample. The most accurate method of determining soil pH is by using a pH meter in soil sample. For this you take a sample of the soil, preferably from different spots, and mix these samples together thoroughly. Take one cup of the mixed soil sample and add 1.5 cups of de-mineralized water. Mix this well and leave it to stand for approximately 1 hour.
Filter the mixture into a clean vessel to separate the water from the solids. You can measure the pH in the water. Paper test strips are probably the least expensive way to check the pH of a nutrient solution. These paper strips are impregnated with a pH sensitive dye which changes color when dipped into the nutrient solution. Universal indicators will change color to indicate a range of pH levels from about pH 2 to pH 10. You can compare the color with a standard color chart to see what pH the solution is to the nearest whole number. The paper test strips are inexpensive, but they can be harder to read.
Test kits
Liquid pH test kits are also available. You add a few drops of a pH-sensitive dye to a small amount of the nutrient solution and then comparing the color of the resulting liquid with a color chart. This method is slightly more expensive than the paper test strips, but it is easier to read, reasonably accurate and reliable. A more high-tech and accurate way to check pH is to use a digital meter. These meters come in a wide range of sizes and prices. One of the more affordable types of pH meter is the digital pen. These pens are manufactured by several different companies and are very handy and easy to use. You simply dip the electrode into the nutrient solution for a few moments and the pH value is displayed on an LCD screen.
pH meters are fast and accurate. However they need to be cared for properly and calibrated to maintain their accuracy. The meters usually need calibrating frequently because they can drift and to ensure accuracy you also need to check them often. The glass bulb electrode must be kept clean, and in some models they must be kept wet at all times. The pH meters are actually very sensitive volt meters and are susceptible to problems with the electrode.
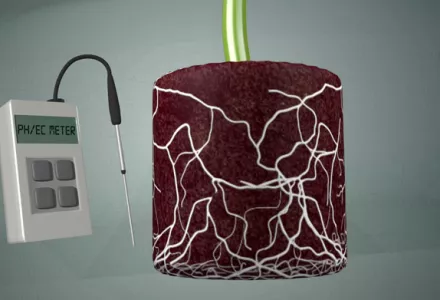
Because pH meters have a reputation for stopping working without warning, it is a good idea to keep an emergency back-up method for checking pH (paper test strips or a liquid pH test kit), just in case. Also watch the video: How to measure pH & EC from the roots?
How can you adjust pH levels?
The pH scale is logarithmic, which means that each unit of change equals a tenfold change in the concentration of hydrogen or hydroxide ions. To put this another way, a pH 6.0 solution is 10 times more acidic than a pH 7.0 solution, and a pH 5.0 solution would be 10 times more acidic than the pH 6.0 solution and 100 times more acidic than the pH 7.0 solution. This means that if you want to adjust the pH of your nutrient solution by 2 points (for example from 7.5 to 5.5), you would have to use 10 times more adjuster than if you were moving the pH value just 1 point (7.5 to 6.5).
Always add the nutrients to the water before checking and adjusting the pH of your solution. The nutrients will usually lower the pH of the water due to their chemical make-up. After adding the nutrients and mixing the solution, check the pH using whichever method you prefer and decide whether you need a product to raise or lower the pH. Add small amounts of pH adjuster. Stir very well and check the pH again. Repeat the above steps until the pH reaches the desired level.
The pH of the nutrient solution can be adjusted by adding acids or alkalis. Products used to raise pH are generally based on 2 alkaline ingredients: caustic potash or potassium carbonate. Potassium carbonate has a buffering effect when used to adjust pH compared to caustic potash. Using caustic potash will cause fluctuations in the pH levels. Using potassium carbonate results in fewer fluctuations and a more steady pH due to the bicarbonate that is in the potassium carbonate. Products used to lower pH are always acids. Nitric acid, phosphoric acid or sulphuric acid can all be used, and these acids contain nitrate, phosphate or sulphur, respectively. It depends on the growing stage of the plant which product is the best to use. Most commonly, nitric acid is used when the plants are in the vegetative growth phase. Phosphoric acid is used when the plants are in the flowering stage.