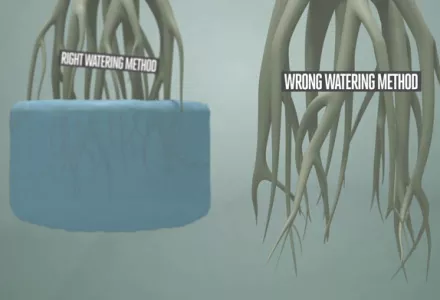
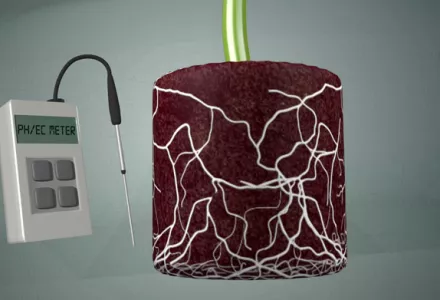
The pH of the soil and the nutrient solution are essential aspects of a good feeding plan. pH does not have a direct effect on the plant, but it does directly affect the availability of the nutrients for the plant. The plant, in turn, can also influence the pH of the soil in the area close to the roots, as we will discuss later in this article.
To better understand the effect of pH on your crop yields, we first need to define pH. The pH scale, the standard measurement of acidity, was developed by the head of Carlsberg Laboratory’s Chemical Department in 1909. It basically means ‘the power of hydrogen’, because the scale provides a simple and universal measurement of the amount of hydrogen ions there are in a solution. These hydrogen ions affect its acidity and how the solution will react chemically. pH is defined as the negative logarithm of the hydrogen ion concentration. It is a result of the presence of anions (negatively charged nutrients) and cations (positively charged nutrients).
The pH scale goes from 0 (acid) to 14 (alkaline) with pH 7 as the neutral point.
The plant can influence the soil life in its rhizosphere
The rhizosphere is the narrow region of soil that is directly influenced by root secretions and associated soil microorganisms. Plants respond to nutrient deficiency by altering their root morphology, recruiting the help of microorganisms and changing the chemical environment of the rhizosphere. Components in root exudates help plants to access nutrients by acidifying or changing the redox conditions within the rhizosphere or chelating directly with the nutrient. Exudates can liberate nutrients via the dissolution of insoluble mineral phases or desorption from clay minerals or organic matter, whereby they are released into the soil in solution and can then be taken up by the plant.
When preparing a nutrient solution, a grower ensures that the pH of the water is within a certain range. This range will preferably be that at which most nutrients are available to the plant, which is 5.2 - 6.2. If necessary the pH of the fertilizer solution can simply be adjusted by adding an acid to lower the pH or a base to increase it (read more about it in pH acidity: what it does to your plants). But in the rhizosphere, the direct surrounding of the living roots, things become very different. The roots excrete many substances that alter the pH in the substrate.

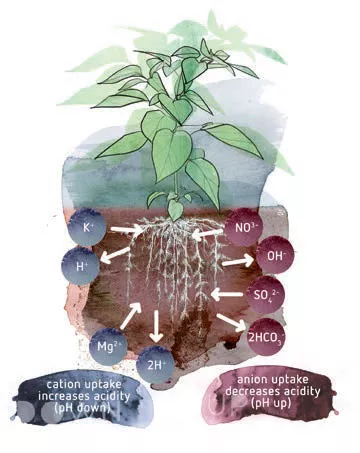
The pH in the rhizosphere can be very different from the pH which is measured in the nutrient solution. The principal cause of this is that the plant has to remain ‘neutral’. When they are dissolved in water, all nutrients are present as ions. Those ions always have a positive or a negative charge. Positively charged ions, like K+, are called cations. Negatively charged ions, like NO3-, are called anions. Some nutrients can be present in multiple forms. For example phosphates, which can occur as PO4 3- , HPO4 2- and H2PO4-. However, only this last form can be taken up by the roots. The surface of the root is negatively charged. In this state, the negatively charged ions such as H2PO4- will be repelled from the root surface like two magnets that have the same pole.
Plants have developed several ways of facilitating anion uptake. For every anion the plant takes up, it excretes an anion such as a hydroxide (OH-) or bicarbonate ion (HCO3-). Similarly, for every cation it takes up, the plant excretes a cation as a H+. In this way, the plant’s charge remains balanced. However, a side-effect of this is that the excreted ions influence the pH of the rhizosphere in the substrate. By excreting a cation, the pH near the roots decreases (it becomes more acid). The excretion of anions will raise the pH near the roots (it becomes more alkaline).
It is well-known that nitrogen fertilizers have an effect on the pH near the roots. That insight is important because the plant takes up so much nitrogen that the effect can be considerable. But this effect occurs with every nutrient or fertilizer.
As a grower, you can add nitrogen in different forms. Ammonium (NH4+) has an acidic effect in the soil. Nitrate (NO3-) has an alkaline effect. One might easily assume that the answer to this is to fertilize with ammonium nitrate (NH4NO3). But it isn’t that simple. The ammonium will be taken up much faster by the plant compared to nitrate, and the result in the end will be acidification of the soil. All these reactions need to be taken into account because every nutrient has its own optimum pH-range in the soil with respect to plant availability. For some elements, this is a narrow pH-range and simply measuring the pH in the nutrient solution will not tell you what is really happening down in the rhizosphere.
Exudates
In the past, it became clear that roots excrete many substances in order to influence the soil-life directly around the surface of the roots. These substances are known as ‘exudates’. The main exudates are sugars and organic acids. Acids such as citric acid, oxalic acid and malic acid are present to a large extent in the cell moisture of the roots. These elements also can have an influence on the pH in the soil but how strong this influence is will vary in every plant.
If the acids are excreted from the roots, they are dissolved as anions and will make the soil near the root more alkaline, like other anions. Usually these exudates will have a minor influence on the pH compared to the strong effect of H+ -ion excretion. What is remarkable, however, is that not every piece of the root system acts in the same way. At the tip of the root, more H+ -ions are excreted, while a bit further down the root, more anions are excreted. This is probably connected to the differences in the uptake of fertilizers.
pH levels affect the availability of nutrients and the growth of the plants
The pH level influences the availability of nutrients and, indirectly therefore, has an effect on the growth of the plants. pH can also affect the absorption of nutrients by plant roots. Not every nutrient is affected equally, but most nutrients are available for plants in the pH range of 5.2 – 6.2 (see Figure 4).
Before a nutrient can be used by the plant, it must be dissolved in the soil solution. Most minerals and nutrients are more soluble, and thus available, in slightly acid soils than in neutral of slightly alkaline soils.
In neutral to slightly alkaline soils some elements can become ‘inactivated’ and will no longer be available to the plant. These elements include iron, manganese, copper, zinc and boron. In very acidic soils, on the other hand, the solubility of phosphorus, calcium and magnesium solubility decreases. Phosphorus is never readily soluble in the soil but is most available in soil with a pH range of around 6.5. This value varies between different substrates.
Extremely acid soils (pH 4.0-5.0) can have high concentrations of soluble aluminum, manganese and iron, which may be toxic to the growth of some plants. Nutrients for healthy plant growth are divided into different categories: macro nutrients (elements needed in larger amounts), which are also subdivided into primary and secondary nutrients and micro nutrients or trace elements (elements needed in very small amounts). Most secondary nutrients and micro nutrient deficiencies can be corrected easily by keeping the medium around the optimum pH range.
Low pH values (3-5) in combination with a high temperature (above 79°F) can also influence the growth of some fungal diseases.
In highly acidic soils the activity of the bacteria that decompose soil organic matter can be impeded. This prevents organic matter from breaking down, resulting in an accumulation of organic matter and the non-release of nutrients into the soil, particularly nitrogen, which is locked inside the organic matter. As a result, the growth of the plants can be negatively affected.
In organic soil substrates, there are beneficial fungi called mycorrhizae. These microorganisms prefer a slightly acidic environment for optimum growth.
The alkalinity of the water is also a relevant factor. If the alkalinity of the water is over 200-250ppm CaCO3, then acid should be added to minimize the influence on the pH of the growing medium.

How and why the pH often changes in hydroponic growing systems
The uptake of anions (negatively charged nutrients) and cations (positively charged nutrients) by plants can cause substantial shifts in pH in the growing system. If more cations are absorbed in relation to anions, the pH will decrease. If more anions are absorbed than cations, this leads to an increase in pH. Since nitrogen (an element required in large quantities for healthy plant growth) can be supplied either as a cation (ammonium - NH4+ ) or as an anion (nitrate - NO3- ), the ratio of these two forms of nitrogen in the nutrient solution can a major effect on both the rate and the direction of pH changes over time. Shifts in pH can occur surprisingly rapidly. Most varieties of vegetable grow best in a nutrient solution with a pH between 5.2 and 6.2 and at a temperature between 68°F and 72°F.
The effect of light spectrum on plant development
When little light is available (read: The effect of light spectrum on plant development), plants will absorb more potassium and phosphorous from the nutrient solution, increasing the acidity (pH will drop). At low light levels the transpiration rate is also lower, which in turn decreases calcium uptake. In combination with a low pH in the substrate, symptoms of calcium deficiency may appear. When there is plenty of intense light (on clear sunny days), plants will take up more nitrogen from the nutrient solution. As a result, acidity decreases (pH rises).
What happens if pH is too high or low and how to recognize symptoms?
The first symptoms of a nutrient deficiency will show up in the leaves. An iron (Fe) deficiency, for example, can occur very fast. At pH values of 7 or over, less than 50% of the Fe will be available to the plants. At pH values of 8.0, just a tiny amount of Fe is left in solution due to iron hydroxide precipitation (Fe(OH)3- which eventually converts to rust).
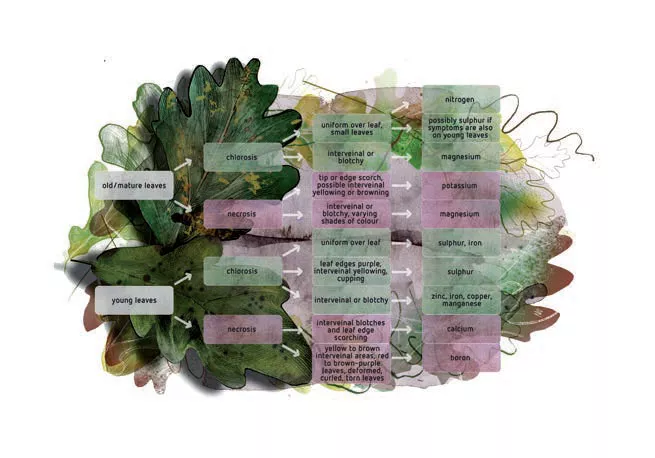
Figure 5 can be used as a tool to identify nutrient deficiencies in plants. Chlorosis is the yellowing or bleaching of green plant tissue due to the loss of chlorophyll. Necrosis is the death of plant tissue and shows as a dark brown discoloration e.g. on a portion of the leaf.
The place on the plant where the symptoms occur (old versus young leaves) will depend on the mobility of the element in the plant. Elements with very low mobility are boron, calcium, copper, iron, manganese, molybdenum and zinc. Deficiencies of these elements will first be seen in the younger leaves. These elements are transferred with the sap-flow to the young leaves. They do not move around within the plant. More mobile elements are nitrogen, potassium and magnesium. Deficiency symptoms of these elements are seen in the older leaves of the plants because the elements relocate from the older leaves to the younger leaves, which need more nutrients for the growing process.