Most growers know the importance of applying the right amount of macro- and micro-nutrients, and there are several ways of knowing whether a plant is lacking any of these elements. However, some of these deficiencies – or excesses on occasion – are not caused by a shortage of the element in question but rather by a poor combination with other nutrients, either in the potting mix, in the plant or both. In this article, we are going to look at the importance of the interaction between different nutrients and how it can affect the final crop.
In 1953, D. Mulder published his “Les elements mineurs en culture fruitière”, one of the first studies of how different nutrients interact. The study included a graph, which is now commonly used. Over the years, other researchers have added other possible synergies and antagonisms. Clearly, studying the interactions between nutrients is essential for improving crop yield.
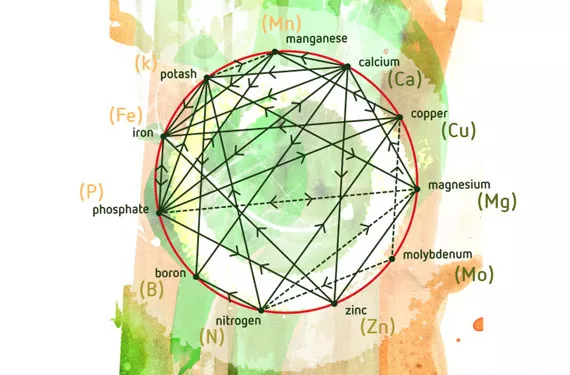
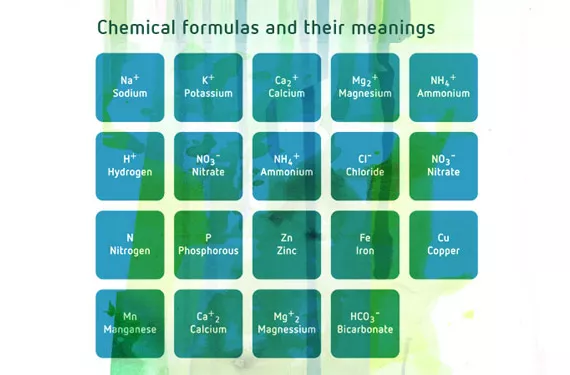
The relative proportions of different nutrients has a direct effect not only on plant nutrition, but also on the substratum in which the plant grows. Cations (positively charged elements) are to a greater or lesser extent retained by the negative charges in certain soil components, such as clay and organic matter. Cations include Na+, K+, Ca2+, Mg2+, NH4+ and H+ (sodium, potassium, calcium, magnesium, ammonium and hydrogen).
Plants absorb elements that are dissolved in water, which means that elements trapped in the soil cannot be used directly. In some cases, however, these elements can filter into the water in the substratum and thus be assimilated by the plant.
The more cations that the soil or substratum can hold, the greater its ‘Cation Exchange Capacity’ (or CEC). The proportion of cations in the soil directly influences the texture of the soil or substratum.
Nitrogen

When in the form of ammonium, NH4+, nitrogen interacts negatively with the plant’s uptake of calcium, magnesium and potassium, particularly when the NO3- (nitrate)/NH4+ (ammonium) ratio is low.
As a result, excess NH4+ can lead to a deficiency in any of these three elements. This is an important problem in hydroponic growing, which normally uses an inert growing medium with a low or zero CEC index; here the quantity of available calcium, magnesium and potassium depends solely on what is in the nutrient solution, unlike soils or substrata with high CECs which normally hold a large quantity of these elements.
There is also an antagonistic interaction between the anions Cl- and NO3- . Excess Cl- (very common in saline and/or sodic water) can reduce the plant’s absorption of NO3- .
The N/K ratio is also crucial when plants are passing from the growth (vegetative) phase to the generative (flowering or fruit-bearing) phase. The primary stimulus for a short-day or long-day plant to go from vegetative to generative is the number of consecutive hours of darkness. However, other stimuli, such as the N/K ratio, also affect these phenological states to some extent.
Fruit contains an abundance of potassium, and it is therefore essential to ensure a proper supply of potassium during generative periods. Yet regardless of how much potassium there is, if the ratio to nitrogen is too low, this can lead to a reduction in flower formation and plants with many vegetative parts (leaves and branches) and few generative parts (flowers and fruit).
Potassium

It is essential to get the proportion of potassium right, since it interacts both in the soil and in the plant with phosphorus, sodium, calcium and magnesium.
In clay soils with a high CEC, when the plants are irrigated with fertilizer solutions in which the potassium is dissolved in its ionic form, some of the potassium is adsorbed by the mineral and humic parts of the soil.
If you irrigate with a low-potassium solution, the potassium held in the soil is released for uptake by the plant. This exchangeable potassium and the solution are known as available potassium. As its name suggests, it is this kind that the plant absorbs most readily.
However, the potassium also comes in non-exchangeable forms which are strongly fixed to the soil components. In this case, it is not directly available to the plant and only enters into the solution when levels of exchangeable potassium are very low. The problem of using this potassium is that it takes a long time to go from its fixed state to the interchangeable state, which means that it is not readily absorbed by the plant.
Applying too much calcium and magnesium can cause a potassium deficiency; the K/Ca and K/Mg ratio should always be kept above 2 (but below 10, since too much K can hinder the absorption of calcium and magnesium). Too much potassium can also prevent the absorption of certain micro-elements, such as zinc. It is particularly important to take account of this interaction when using very hard water with a high calcium and magnesium content.
Phosphorus

An excess of phosphorus interacts negatively with the majority of micro-elements (Fe, Mn, Zn and Cu). In some cases, this is due to the formation of insoluble precipitates and in other cases, to metabolic processes in the plant which prevent the transfer of the nutrient from the root to other parts of the plant. This is the case, for example, with the P/Zn interaction. The P/Fe interaction appears to be negatively regulated at the cellular level and by the formation of insoluble complexes. The P/Cu interaction normally involves the formation of precipitates in the root area.
Genetic interactions can vary from one species to another and even between different varieties of the same species. For example, in some species a positive effect has been observed between the amount of available phosphorus and the plant’s resistance to salinity, meaning that an increase in this element leads to greater resistance. Other studies, however, conclude that the effect is negative.
There have also been reports of a reduction in the availability of sulfur and calcium when large quantities of phosphate are applied. In the case of calcium, this is caused by the formation of insoluble phosphates.
In contrast, phosphorus favors the absorption of magnesium, so a shortage of phosphorus could also lead to a magnesium deficiency if the latter is present in small quantities.
Both NO3- and NH4+ facilitate the absorption of phosphorus. In the case of NH4+, the reason appears to be the excretion of H+ ions by the plant when nitrogen is administered in this form in significant quantities. These H+ ions cause a slight acidification of the root area, which can favor the solubility of some phosphorus salts which would otherwise be trapped or remain in an insoluble form.
Magnesium

It is also important to take account of the Ca/Mg ratio. Its most important effect is its influence on the soil structure. Calcium in the soil tends to improve aeration, while Mg favors the adhesion of soil particles. Thus, if the Ca/Mg ratio is very low, which means that much of the exchange complex will be occupied by these Mg ions, the soil becomes less permeable, harming the development of the crops. Because of this, the Ca/Mg ratio should always be kept above 1.
This ratio is also important for the mineral balance within the plant. The Ca/Mg ratio in the leaves of some plants is about 2:1, which means that it is necessary to apply greater quantities of calcium than magnesium via the nutrient solution.
Magnesium uptake is also influenced by Zn and Mn levels in the growing medium; an overdose of these micro- elements, as well as being toxic, could also reduce the plant’s absorption.
Interaction of Sodium with Calcium, Magnesium and Potassium
Sodium has a negative effect on most plants due to its toxicity, when it accumulates in certain tissues of the plant, and its capacity to harm the soil structure by competing with other cations for adsorption (the adhesion of the cation to the surface of some soil components). When a soil contains a level of sodium that might prove harmful to crops, it is said to be sodic. Soil sodicity should not be confused with soil salinity, which refers to the total quantity of salts in the soil, without specifying which salts are more prevalent.
There are two ways of determining where there is a risk of harm from excess sodium. One is by calculating the ratio between the sodium and other dissolved cations that will be absorbed by the plant. This is known as the sodium adsorption ratio or SAR. The formula is as follows:
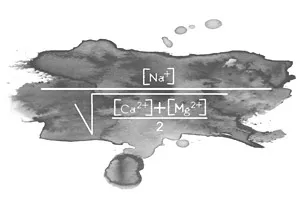
Irrigation water with a SAR over 18 is considered as having a high sodium content.
Another way is by calculating what proportion of sodium cations is retained in the exchange complex, as compared to others. This is known as the exchangeable sodium percentage (ESP).
ESP = 100 x Na / CEC
A soil is considered sodic if it has an ESP of over 15%.
Finally, the ratio between calcium, magnesium and sodium can be altered by the presence of carbonates and bicarbonates. In other words, even if there is initially more Ca and Mg than Na – in principle a good ratio for avoiding problems – if you irrigate with very hard water containing large quantities of carbonates and bicarbonates, they can make the calcium and magnesium precipitate in the form of insoluble carbonates, tipping the scales in favor of sodium and increasing the SAR.
This is known as the residual sodium carbonate (RSC) index. The formula is as follows:
RSC=(CO3-+HCO3-)-(Ca+2+Mg+2)
Tap water with values over 2.5 should not be used, as it can cause problems.