All over the world, questions pour in as to the distinctions between hard water and soft water and how these differences affect how and what plants are fed.
The U.S. department of the interior and the U.S. Geological Survey (USGS) define 60 mg/l (60 ppm) or less of certain ions as soft water. Water with over 120 mg/l (120 ppm) is considered hard, and water in between is moderately hard. Other countries and agencies hold their own distinctions. (See Table 1) Strictly speaking, it is the concentration of dissolved positive multivalent metallic ions with a charge of +2 or +3, typically Calcium and Magnesium. These dissolved elements can and will react with other elements dissolved in water or anything it comes into contact with. Hard water is an issue for cleaning, for equipment, and increases the chemical activity of the water especially where pH is concerned, lending a buffering capacity that can increase the pH over time. As for drinking water, hard water is often considered healthier since it contains mineral nutrients. Typically this comes from ground water that has been exposed for longer periods to mineral bearing rock. Well water is a prime example.
Soft water on the other hand, allows soap to foam up and work better, has less issues for equipment, and provides more of a blank slate in chemical reactions. Studies have shown a correlation between soft water and health issues including cardiac disease. Typically this comes through surface water, rivers, streams, and lakes that have not been exposed to mineral bearing rock formations for long periods. It can also be composed of treated water where most of all ions have been removed by reverse osmosis or have been replaced by single valence atoms such as sodium from water softening equipment.
Table 1. Recommended water quality parameters for greenhouse irrigation (Penn State University).
| Parameter | Level of concern |
|---|---|
| Electrical Conductivity (EC) | Above 1.0 mmhos/cm (plugs), above 1.5 mmhos/cm (other plants) |
| pH | Below 5.0 or above 7.0 |
| Total Alkalinity (as CaCO3) | Below 30 ppm or above 100 ppm |
| Hardness (Ca and Mg) | Below 50 ppm or above 150 ppm |
| Total Dissolved Solids (TDS) | Above 640 ppm (plugs), above 960 ppm (other plants) |
| Sodium Absorption Ratio (SAR) | Above 2.0 |
| Sodium (Na) | Above 50 ppm |
| Chloride (Cl) | Above 30 ppm (sensitive plants), above 70 ppm (most plants) |
| Nitrate-Nitrogen (NO3-N) | Above 5 ppm may indicate contamination |
| Ammonium-Nitrogen (NH4-N) | Above 1 ppm may indicate contamination |
| Phosphorus (P) | Above 5 ppm may cause deficiencies |
| Potassium (K) | Above 120 ppm |
| Calcium (Ca) | Below 40 ppm or above 100 ppm |
| Magnesium (Mg) | Below 25 ppm |
| Sulfur (S) | Below 10 ppm |
| Iron (Fe) | Above 5 ppm |
| Manganese (Mn) | Above 2 ppm for sensitive plants |
| Boron (B) | Above 0.5 ppm (sensitive plants), above 2.0 ppm (most plants) |
| Copper (Cu) | Above 0.2 ppm |
| Zinc (Zn) | Above 0.3 ppm |
| Fluoride (F-) | Above 1.0 ppm |
| Aluminum (Al) | Above 5.0 ppm |
Problematic ions
Bad Water is bad water, whether because it has a high salt content or has undesired chemicals in it. It can be found anywhere especially in industrial areas, intense agricultural regions, and close to bodies of salt water. This has no bearing on water hardness.
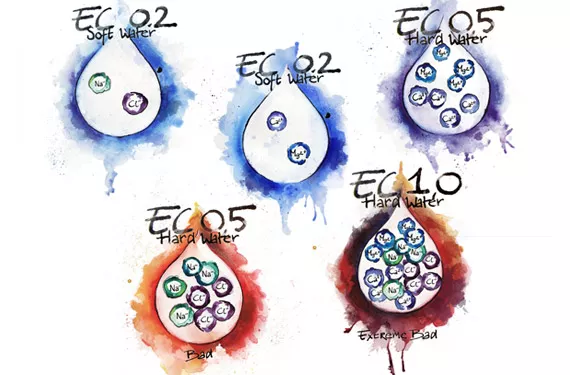
Do not confuse ion or salt concentration with hardness or softness of water. Hardness is a function of multivalent ions like Ca2+ and Mg2+ not monovalent ions like Na+ or Cl+. Monovalent ions also show up in the Total Dissolved Solids (TDS) of a solution, so it is possible to have a TDS of 450 mg/L (1 ppm = 1 mg/L), derived from adding table salt to distilled water, but have soft water. There is no direct correlation between TDS or EC (electrical conductivity) and water hardness unless it is known with certainty that all EC derives exclusively from Ca, Mg or other positive multivalent metallic ions. Sugar water has EC but no hardness. Water softeners work this way by displacing the problem ions Calcium and Magnesium with Sodium ions. The EC stays the same or increases but the water goes from hard to soft; not a good thing for plants.
For us, the big question is “How does this affect the nutrients for plants?” One of the biggest effects for growing systems using hard water is the potential for insoluble deposits of Calcium or Magnesium carbonates. This combining of these ions is an endothermic reaction meaning that as heat is supplied to the solution, the process gets faster. The process of pumping water from a reservoir, through a pump, through smaller pipes, onto a table top and through a root system imparts increasing amounts of heat to the solution so the reaction is natural and persistent.
Reduced flows
At this heat enters the system, the combining of these elements increases, resulting in the deposition of insoluble materials on the inside of pumps, pipes, tubes and medium of the growing system. Ultimately this leads to reduced flows, blocked emitters, burnt out pumps and so on.
The effect on the chemical profile of the nutrient package can also be affected through various antagonistic relationships between individual elements and the overall affect on pH. The harder the water the more Calcium and Magnesium is being applied. The higher these elements get in relation to some other elements like Potassium and Phosphorus, the less available these elements become, effectively locking out these elements. These positive Ions will bring the pH of the solution up, and when hardness is also affected by carbonate levels, the pH effect will continue into the medium to which it is applied. The harder the water, the more acid is required to lower the pH.
There are a couple of commercial solutions to various situations of concentration and hardness. The first is Water Softening. Water softening involves flooding the water with a monovalent ion, typically Sodium, which drives out the Calcium and lowers the hardness of the water. This is great for clothes washing and baths but not so great for consumption by plants and humans, especially where the water is very hard.
The next is Reverse Osmosis (RO), a process where tap water is forced through a series of membranes with progressively smaller pores that block molecules and atoms of a certain size. This filters out the Calcium and other larger elements effectively lowering the water hardness. It also strips out most of all the elements including harmful molecules, Sodium ions, and most other ions thus effectively lowering overall Total Dissolved Solids and EC. It is also expensive to install and maintain and is not always necessary, unless the starting water is very hard or has an excess of harmful elements. It's also possible to put very hard water through reverse osmosis and then mix some of the original tap water back, so long as it is safe tap water. This will regain some hardness for a calcium sensitive system like coco.
Water sample
Decent nutrient companies should take the concerns of hard or soft water into account with the design of their products. Different lines have different needs in this area. Most of these differences are influenced by the medium the product is applied to. Potting mixes have a greater buffering capacity, the ability to hold elements, and should not be recirculated. Recirculating adds more heat to the system and allows deposits to form more readily. Potting mixes have natural buffers that hold pH changes down. The difference in content should be adjusted through the correct ratio of nutrients found in a fertilizer specially developed for potting mixes.
Only soft water is recommended for recirculating systems on inert mediums, so pure RO is acceptable in this system. Recirculating systems have to be able to adjust to, not only the hardness of the water, but also to the additional elements applied in the tap water over and above what is added or needed in the nutrient added. Controlling salt composition is critical because this also affects pH and pH is critical in signaling a plant’s flower response (in addition to photoperiod change). The best would be to use a nutrient which is designed to work with tap water EC values no greater than 0.3 – 0.4 mS/ cm while providing some buffering for pH control in the system (for example CANNA AQUA).
Another situation of current growing systems is the Run-To-Waste system where tank mixed nutrients are applied to a plant and the excess is allowed to drain away and not be re-captured. In this system, it is important to not only adjust the pH once it is mixed, but to maintain that pH across time as the product sits in a prepared tank. This keeps pH swings down while keeping insoluble compounds from forming. Also, there are less Calcium and Magnesium ions available in soft water and the amount needs to be augmented or replaced to achieve the correct disposition of ions. To make growing easier while allowing you to worry less about nutrient composition you should use a nutrient brand that has both a soft water and a hard water version (for RTW) to choose from (like CANNA SUBSTRA). How to know when to use the Hard Water version or the Soft Water version? Simple, see the above explanation and run a water sample.
So, what is gained with this knowledge: the appreciation of the fact that there are many aspects affecting water quality. Not only is the total amount of dissolved ions an issue, but also the composition of these elements and the effect they have on added nutrient packages and the post chemical reactions that can and will occur. Ultimately, it all affects the plant. Nutrients have to be designed and used based on the conditions of the water that the grower intends to utilize as source water. In the end, it is also about correctly designed nutrient packages that allow for both the plant’s nutrient requirements and the long term effect on plant development, and the effect of the medium on composition, storage, and reactivity. Testing is knowing, and knowing is growing; how much do you know?
Bibliography
- Baily, D, T Bilderback, and D Bir. “Water considerations for container production of plants.” North Carolina State University Horticulture Information Leaflet 557. 1996.
- Kessler Jr., J. R. “Water Quality Management for Greenhouse Production.” Alabama Cooperative Extension Service Publication ANR-1158. Alabama A&M and Auburn University, 2005.
- Swistock, Bryan. "Interpreting Irrigation Water Tests." Penn State University Extension Services, 2016.